Kidney
Newsletter
Keeping you up to date with the latest exciting advances and discussions in nephrology
Clinical challenges in kidney disease management
The majority of patients with CKD have mineral and bone disorders.1
This presents as abnormalities in the following parameters:2
 |
 |
 |
 |
 |
SHPT is a progressive disease that manifests frequently in patients with stage 3–4 CKD and from as early as stage 2 CKD2,3
|
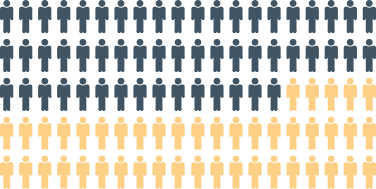
~40–55% of stage 3b CKD patients are affected by SHPT4 |
 |
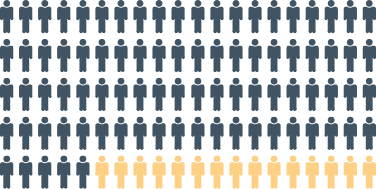
Rising to ~70–85% in stage 4 CKD4 |
SHPT and/or high PTH is associated with an increased risk of:
|
Cardiovascular |
Fractures5,8 |
Progression to dialysis5,7 |
Therapeutic resistance9 |
Death5,8 |
Hyperphosphatemia is particularly common in patients on dialysis (affecting ~40% of these patients)4
|
Uncontrolled serum phosphorus levels
Increased all-cause mortality10 |
Serum phosphorus levels
Increased CV mortality11 |
Few therapeutic agents are licensed for use in the pre-dialysis CKD/SHPT setting17

 |
Nutritional vitamin D
|
 |
Active vitamin D/analogs
|

Dietary phosphate restriction is associated with severe consequences
- Hypoalbuminuria and protein-energy wasting are associated with increased mortality, hospitalizations and a reduced quality of life21–25

The use of phosphate binders in patients on dialysis can:26–28
- Reduce phosphorus levels
- Allow an increase in protein intake
FOOTNOTES
Ca, calcium; CKD, chronic kidney disease; CV, cardiovascular; FGF-23, fibroblast growth factor 23; G, grade, KDIGO, Kidney Disease: Improving Global Outcomes; MBD, mineral and bone disorder; P, phosphorus; PTH, parathyroid hormone;
SHPT, secondary hyperparathyroidism.
REFERENCES
- Waziri B, et al. Int J Nephrol Renovasc Dis 2019;12:263–76;
- Cunningham J, et al. Clin J Am Soc Nephrol 2011;6:913–21;
- Wolf M. J Am Soc Nephrol 2010;21:1427–35;
- Levin A, et al. Kidney Int 2007;71:31–8;
- Xu Y, et al. Clin Kidney J 2021;sfab006;
- Arase H, et al. Circ J 2020;84:1105–11;
- Bermudez-Lopez M, et al. ERA-EDTA 2020: Abstract PO878;
- Geng S, et al. Osteoporos Int 2019;30:2019–25;
- Tabibzadeh N, et al. Nephrol Dial Transplant 2021;36:160–9;
- Fernandez-Martin J, et al. Nephrol Dial Transplant 2015;30:1542–51;
- Tentori F, et al. Am J Kidney Dis 2008;52:519–30;
- Roetker N, et al. Am J Nephrol 2019;49:225–32;
- Wetmore J, et al. Kidney Rep 2021;6:1141–50;
- KDIGO. Kidney Int Suppl 2017;7:1–59;
- Strugnell SA, et al. Am J Nephrol 2019;49:284–93;
- Ennis JL, et al. J Nephrol 2016;29:63–70;
- Cozzolino M, Ketteler M. Expert Opin Pharmacother 2019;20:2081–93;
- Bover J, et al. Clin Kidney J 2021;sfab035;
- Cozzolino M, et al. Clin Kidney J 2021. doi.org/10.1093/ckj/sfab091;
- KDIGO. Kidney Int Suppl 2009:S1–130;
- Eriguchi R, et al. Clin J Am Soc Nephrol 2017;12:1109–17;
- Kalantar-Zadeh K, et al. Clin J Am Soc Nephrol 2010;5:519–30;
- Shinaberger C, et al. Am J Clin Nutr 2008;88:1511–8;
- Sarav M, Kovesdy C. Clin J Am Soc Nephrol 2018;13:1558–60;
- Jadeja Y, Kher V. Indian J Endocrinol Metab 2012;16:246–51;
- Fissel R, et al. Hemodial Int 2016;20:38–49;
- Gutekunst L. J Ren Nutr 2016;26:209–18;
- Coyne D, et al. Clin Nephrol 2017;88:59–67.



 BACK TO OPTIONS
BACK TO OPTIONS